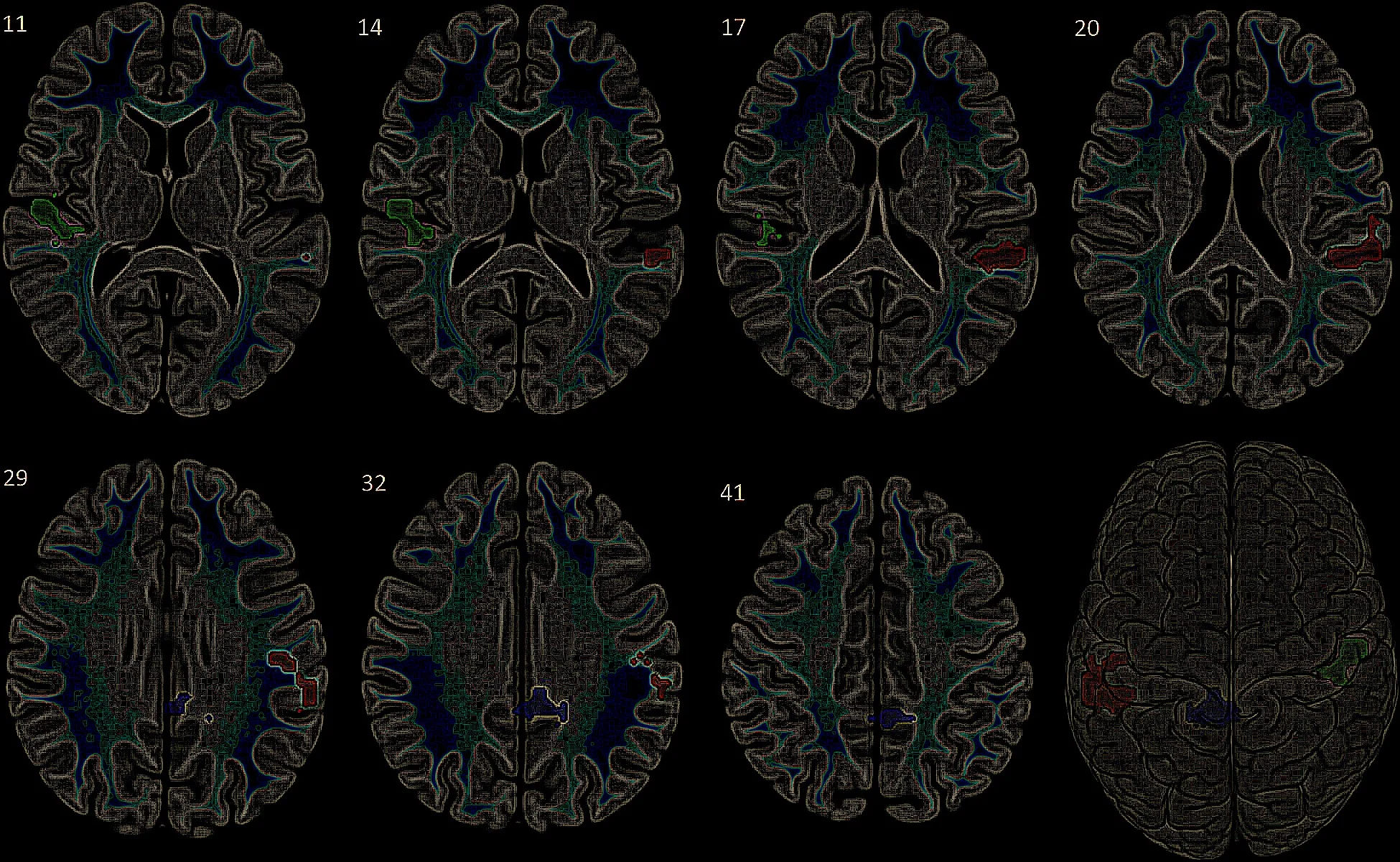
The SCN synchronizes peripheral and central clocks via signaling molecules such as vasoactive intestinal peptide (VIP) and arginine vasopressin (AVP). These oscillatory signals entrain downstream neuronal populations, influencing neurotransmitter systems including GABA, glutamate, dopamine, and serotonin.
In ASD, disrupted SCN signaling manifests as altered sleep–wake cycles, diminished melatonin secretion, and abnormal cortical oscillations. Melatonin pathway disruption is particularly significant, as many ASD patients carry polymorphisms in the AANAT or ASMT genes, which encode enzymes in melatonin synthesis. At the molecular level, melatonin acts via MT1 and MT2 G-protein coupled receptors to modulate cAMP/PKA signaling and Ca2+ channel activity, both of which influence synaptic transmission. Drug candidates that act as MT2-selective agonists (e.g., tasimelteon) may offer circadian phase-specific correction of excitatory–inhibitory imbalance in ASD.
Neurotransmitter regulation by circadian rhythms is also tightly coupled with molecular action. For instance, CLOCK-BMAL1 heterodimers regulate expression of tyrosine hydroxylase, controlling dopamine synthesis. Given dopamine’s role in social motivation, drugs aligning dopaminergic rhythms with SCN output could have therapeutic potential.
Peter De Ceuster. (2026). Integrating Circadian Rhythm and Neurobiology in Precision Medicine: A Novel Approach for Treating Severe Autism Through the Exploration of Time Cells and Genetic Mechanisms
Excerpt from: Integrating Circadian Rhythm and Neurobiology in Precision Medicine: A Novel Approach for Treating Severe Autism Through the Exploration of Time Cells and Genetic Mechanisms by P. De Ceuster
© All rights reserved. Do not distribute. (Publication: Integrating Circadian Rhythm and Neurobiology in Precision Medicine: A Novel Approach for Treating Severe Autism Through the Exploration of Time Cells and Genetic Mechanisms)